
Natural Product Pathways in Plants and Single Cells
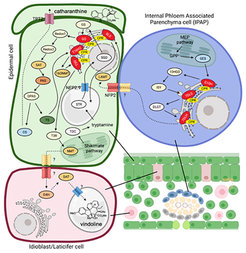
Natural products are synthesized through complex metabolic pathways by the sequential action of dedicated enzymes that are located in different cell compartments and in different cell types. Thus, the correct assembly of the final products requires a highly coordinated action of enzymes, transporters and accessory proteins across different cell types within the tissue.
To understand these complex processes, we are implementing and adapting the latest single-cell ‘omics sequencing technologies for the analysis of our plants of interest. We are also developing mass spectrometry-based methods for targeted and untargeted analysis of plant natural products in single-cells. We are currently applying these new approaches to answer open questions in alkaloid biosynthesis.
Biosynthesis of vinblastine in Catharanthus roseus
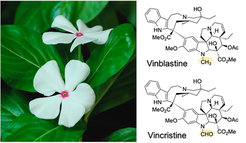
The medicinal plant Catharanthus roseus (Madagascar periwinkle) is considered Africa’s gift to human kind because it is the sole source of two anticancer drugs, vinblastine and vincristine, which have been used for more than 60 years. The biosynthesis of these dimeric metabolites, called bisindoles, requires more than 30 chemical reactions, most of which have been elucidated over the past three decades. These chemicals derive from the dimerization of two monoterpene indole alkaloids (MIAs), catharanthine and tabersonine. However, the mechanism of the dimerization and the further derivatization of the coupling product is still unknown. We are using a combination of transcriptomics, metabolomics and biochemistry at tissue and cellular level to unveil the final biosynthetic steps in the assembly of these precious natural products.
Single-cell metabolomics (scMet)
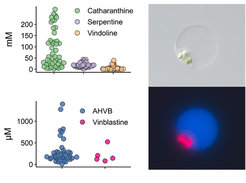
Analysis of the metabolic profile of single cells is not routinely utilized. Reported scMet methods lack sensitivity and the possibility for rigorous structural identification. Therefore, we have developed a method for single-cell metabolomics (scMet) that allows analysis of 180 cells/day, providing both qualitative and quantitative data of the metabolites detected in each cell. We have applied this method to quantify many classes of natural products from single cells of C. roseus and other plants. This method allows us to identify in which cell types different biosynthetic reactions take place, to map transport of the biosynthetic intermediates and final products, and to ultimately develop models for the role that localization plays in controlling in plant metabolism. In combination with single-cell transcriptomics, scMet will allow us to tackle some of the most cryptic metabolic steps in natural product biosynthesis.
Evolution of metabolic pathway partitioning
Advances in DNA and RNA sequencing technologies have given us access to information to identify genes involved in natural product biosynthesis. Typically, we compare bulk RNA transcriptome and metabolite analysis of different to identify differentially expressed genes that are correlated to different levels of metabolites in the tissues. This approach is effective but not efficient, as hundreds of genes must be screened before the correct one is found.
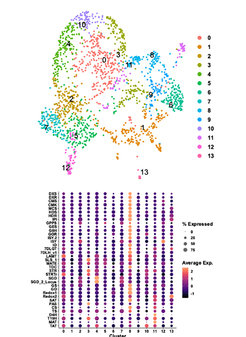
The recent application of single-cell RNA sequencing methods to plants is changing the landscape of plant gene discovery. Thus, we are establishing pipelines for generating in-house single-cell transcriptome data, along with developing methodology to obtain deeper sequencing of single cells of interest. These datasets will facilitate the identification of genes for natural product pathways of interest. Additionally, these datasets will reveal the evolution of cell type specificity, showing whether partitioning of specific biosynthetic pathways between different cell types is conserved across related plants.
Transport of metabolites in alkaloid biosynthesis

MIA biosynthesis in C. roseus exhibits a sophisticated spatial organization across at least three distinct cell types and seven subcellular locations. This compartmentalization limits the production of bisindole alkaloids in C. roseus, posing challenges for their reconstitution in foreign hosts. However, the mechanisms underlying the transport of MIAs remain largely unexplored. We aim to discover and characterize the cellular and subcellular membrane gatekeepers of MIA biosynthesis in C. roseus.
Biosynthetic potential of plant cell cultures
Plant cell cultures are used commercially for controlled production of useful secondary metabolites. However, plant cell cultures often produce valuable compounds at low levels, and display high levels of genetic and epigenetic instability. The single cell methods that we are developing allow us to unravel the mechanistic basis this instability, and how these cultures can be engineered for the production of high value metabolites, such as the anticancer drug vinblastine.





