Chemical Ecology of Plant-Pathogen Interactions
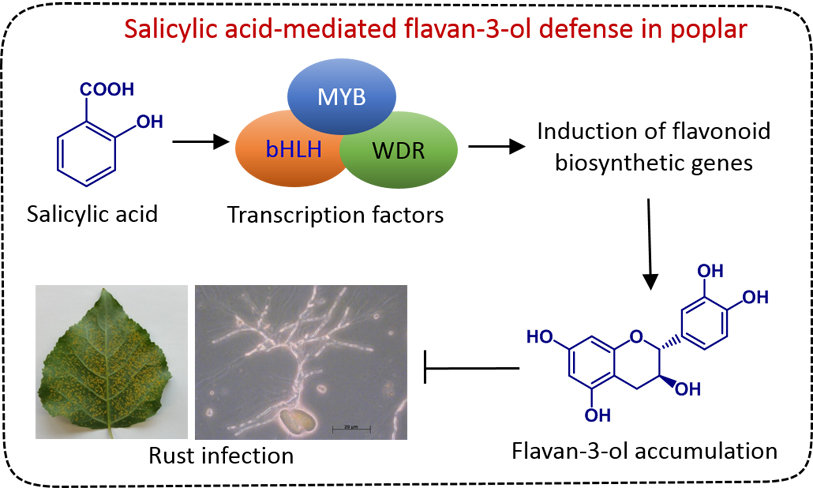
In nature, plants are subjected to a diverse range of harmful pathogens and environmental stresses. To cope with those stresses, plants possess sophisticated defense or tolerance mechanisms. Every plant cell has the potential of innate immunity enabling prompt and efficient response towards pathogens. In addition, the localized immune responses can also be transmitted over a long-distance making the entire plant well protected against subsequent attacks. Small molecules, either conserved across the plant kingdom (e.g., hormones) or synthesized by specific plant taxa (e.g., specialized metabolites), play a significant role in plant defense. In our group, we are interested in understanding the chemistry, biosynthesis, regulation, and functions of several classes of plant metabolites. We use a range of analytical, biochemical, molecular, and ecological approaches to understand how low-molecular-weight plant metabolites mediate the outcome of host-pathogen interactions, especially in poplar trees and rice.

Project 1: Biochemistry and signaling of strigolactones in poplar-pathogen interactions
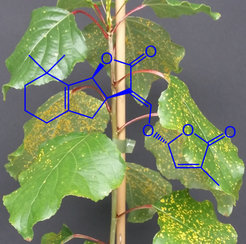
Strigolactones (SLs) are a relatively new class of terpenoid hormones that regulate diverse aspects of plant development and symbiotic plant-microbe interactions. Historically, root-secreted SLs were identified as germination stimulators of parasitic weed seeds. More than 25 naturally occurring SLs have already been discovered in several monocot and dicot plants, and this number will likely increase in the near future. The presence of SLs at very low concentrations in plants and their structural diversity makes these compounds challenging to quantify. Since the recognition of SLs as a new plant hormone, many investigations have been carried out on their chemistry, biosynthesis, signaling, and functions in several plant species. However, the role of SLs in plant-pathogen interactions remains controversial. Furthermore, our current understanding of the role of SLs in tree biology is largely unknown, despite most of the genes involved in SL biosynthesis and signaling having been reported in poplar. This project is designed to survey the SL structures produced in poplar, characterize the key enzymes, determine their roles in defense against pathogens and plant development, study the potential interactions with other hormones and determine whether SLs regulate secondary metabolism.
Project 2: Biosynthesis and functions of caffeoylquinic acids in poplar
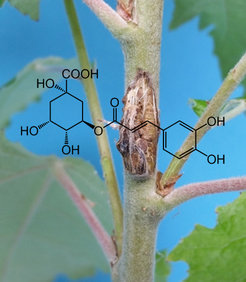
Caffeoylquinic acids (CQA) are esters of caffeic acid and quinic acid accumulated in many plant species. These phenolic compounds are products of the lignin pathway and are known to serve various ecological functions. In addition, the presence of CQAs in plant-based human diets reduces the risk of cardiovascular disease and cancers. CQAs can vary structurally through the attachment position of the quinic acid moiety to the caffeoyl moiety. For example, many plants are known to accumulate 5CQA, 4CQA, 3CQA and 1CQA. All of these structures are collectively called chlorogenic acids. Poplar synthesizes and accumulates substantial amounts of 5CQA and 3CQA in leaf, bark and root. We have found that these metabolites are differentially induced in poplar leaf and stem upon pathogen attacks. Now the question arises why plants produce multiple CQAs? Since we know that they are induced under different conditions, do they have different functions? Do they work as anti-oxidants or pro-oxidants? All these research questions made these compounds extremely interesting to study.
Project 3: Exploration of natural resistance in rice against pathogens: a focus on terpenoid phytoalexins and their hormonal regulation
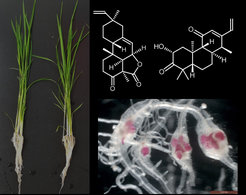
Phytoalexins are low-molecular-weight antimicrobial secondary metabolites that are synthesized de novo and rapidly accumulate in plants after pathogen attacks. Phytoalexins are usually distributed in certain plant families, such as camalexin in the Brassicaceae, stilbenoids in the Vitaceae and the Fabaceae, diterpenoids in the Poaceae, and coumarins in the Solanaceae. While research on camalexin and stilbenes has proceeded rapidly, we know much less about the biochemistry and functions of other phytoalexins. Rice (Oryza sativa L) is an important cereal crop, which synthesizes diverse diterpenoid phytoalexins, including various oryzalexins, phytocassanes, and momilactones. However, the composition and content of phytoalexins differ remarkably among rice cultivars. Screening of a big collection of rice accessions from different parts of Asia revealed that the genotype KPM confers resistance against the root-knot nematode Meloidogyne graminicola. A non-targeted metabolomics analysis hinted that KPM synthesizes several diterpenoid phytoalexins in very high amounts compared to the susceptible Nipponbare (unpublished). These results are very intriguing as these compounds have not been studied against root pathogens. This project aims to elucidate the role of these metabolites against root-knot nematodes. We are also interested in exploring how the induction of diterpenoid phytoalexins is regulated.
This project is in collaboration with Prof. Godelieve Gheysen, Ghent University, Belgium.