Biosynthesis and Function of Volatile Formation in Woody Plants and Grasses
Many plants respond to herbivore feeding by producing complex volatile blends. These blends consist of compounds from several chemical classes such as terpenoids, aromatic esters, and green-leaf volatiles. We are investigating the biochemical and genetic basis of herbivore-induced volatile production in woody plants and grasses. Gaining basic knowledge about these processes will help us to elucidate the biological role of single volatile compounds in plant-insect interactions.
The evolution of insect-induced terpene biosynthesis in the grasses
Dr. Tobias Köllner
(in collaboration with Dr. Feng Chen, University of Tennessee)
The aim of this project is to investigate the molecular basis of insect-induced terpene biosynthesis in sorghum and the evolution of responsible terpene synthases in different grasses. Currently we identified three terpene synthase genes from sorghum which showed increased expression after herbivore-feeding. Biochemical characterization revealed that the three TPS produce the same sesquiterpenes but with completely different product distributions. Comparative studies with terpene synthase orthologs from sorghum, rice and maize will help us to identify key amino acids which changed during evolution and resulted in altered product specificities.
Biochemical and functional aspects of the metabolism of DIMBOA-Glc in maize and wheat
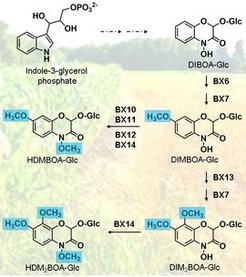
Benzoxazinoids (BXDs) are plant secondary metabolites that are well known for their defensive and allelopathic properties. They are mainly produced in plants of the grass family (Poaceae), including agricultural crops such as maize, wheat, and rye. The biosynthesis of BXDs has been extensively studied in maize and especially the biosynthetic pathway for the benzoxazinoid hydroxamic acids is fully elucidated in this plant. The core pathway starts from indole-3-glycerol phosphate, which undergoes several oxidations, a glucosylation, and a methylation leading to 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one glucoside (DIMBOA-Glc). DIMBOA-Glc is the most abundant BXD in undamaged maize, however, after herbivore feeding it is converted to a variety of other compounds such as HDMBOA-Glc, DIM2BOA-Glc , and HDM2BOA-Glc. In collaboration with the groups of Dr. Georg Jander (Boyce Thompson Institute, Ithaca, USA) and Prof. Dr. Matthias Erb (University of Bern, Switzerland), we have recently identified and characterized the enzymes involved in the formation of HDMBOA-Glc, DIM2BOA-Glc, and HDM2BOA-Glc in maize (see figure). The aim of the present study is to identify respective enzyme homologues in wheat and to investigate how they might have evolved. Preliminary BLAST analysis with the wheat genome revealed no direct orthologues, suggesting a convergent evolution of DIMBOA-Glc metabolism in the grasses. Since fungus infestation can induce the conversion of DIMBOA-Glc into other BXDs in wheat, a transcriptomic dataset derived from non-infested and fungus-infested wheat plants will help us to identify upregulated candidate genes. Further on, we are interested in elucidating the biosynthesis of benzoxazinoid lactams such as HMBOA-Glc in both maize and wheat. These compounds are frequently found in the leaves besides benzoxazinoid hydroxamid acids, but the enzymes responsible for their formation are still unknown and the biological roles of these compounds are unclear.
