Neurophysiology
Functional Imaging
We use widefield as well as 2-photon functional imaging to monitor the neuronal activity of single neurons or whole neuronal populations simultaneously. For functional imaging experiments in non-genetic model organisms, as different moths species, we bath apply calcium-sensitive dyes (e.g. Calcium Green-AM) to the insect brain (please see the following papers for further information: Bisch-Knaden et al. J Exp Biology. 2012; Bisch -Knaden et al. Proc R Soc B. 2014). In Drosophila melanogaster several binary transcriptional systems are available, such as GAL4-UAS or LexA-Aop, which we employ to genetically express different fluorescent calcium-sensitive proteins (e.g. Cameleon or G-CaMP). Depending on the promotor line, the calcium sensors can be expressed in selective neuronal population as e.g. olfactory sensory neurons or projection neurons. Please see our review on ‘Calcium Imaging of Neural Activity in Drosophila’ for further details (Strutz et al. Neuromethods. 2012).
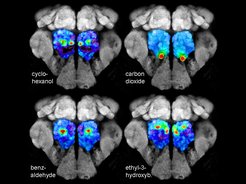
GC-Functional Imaging
Odor information is predominantly perceived as complex odor blends. In order to analyze and monitor how natural odor blends are coded and processed by the olfactory circuitry, we developed a technique that combines gas chromatography and functional imaging (GC-I). This technique allows to identify the active odor components of a complex blend, use these compounds as stimuli in close to natural occurring concentrations and to measure odor-induced calcium signals in selective neuronal populations of the insect olfactory system. The functional GC-I technique can be seen as a valuable complementary method to classical GC/electrophysiology techniques and represents a highly useful tool to study insect-insect and insect-plant chemical interactions.
Patch Clamp
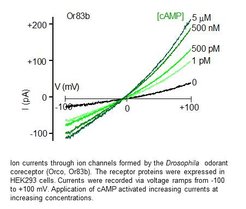
Olfactory receptors in insects are ion channels that are activated by the binding of odor molecules. In order to investigate the biophysical and pharmacological properties we register the currents passing these channels using the patch clamp method. This is an electrophysiological technique that allows to measure tiny ion currents across the plasma membrane of cells or membrane patches. These measurements can be performed with olfactory receptor neurons that express these receptors endogenously or in culture cells transfected with the receptor-coding DNA (Wicher et al. Nature. 2008).
Cell Culture
To express insect olfactory receptors or other ion channels we use cultured cells such as human embryonic kidney (HEK293) cells or Chinese hamster ovary (CHO) cells. Cells plated in a dish are transfected with the plasmids that carry the genetic information to produce the proteins of interest (Halty-de Leon et al. J Neurosci Methods. 2016). The functional properties of these proteins can be investigated using electrophysiological or fluorescence optical methods.



